Quantum dots can achieve a lot of things. These are material structures, and are only a few nanometres in size. Their behavior is quite similar to that of molecules or atoms- their size, number of electrons and form can be modulated systematically, which means that the customization of the electrical and optical characteristics for various target areas is possible, like for new display technologies, biomedical applications and as well as photovoltaics and photocatalysis.
There is another, current line of application-oriented research which aims to produce hydrogen directly form water and solar light because hydrogen is a clean and much more efficient energy source. It can be converted into other, widely used forms of fuel, such as methanol and gasoline.
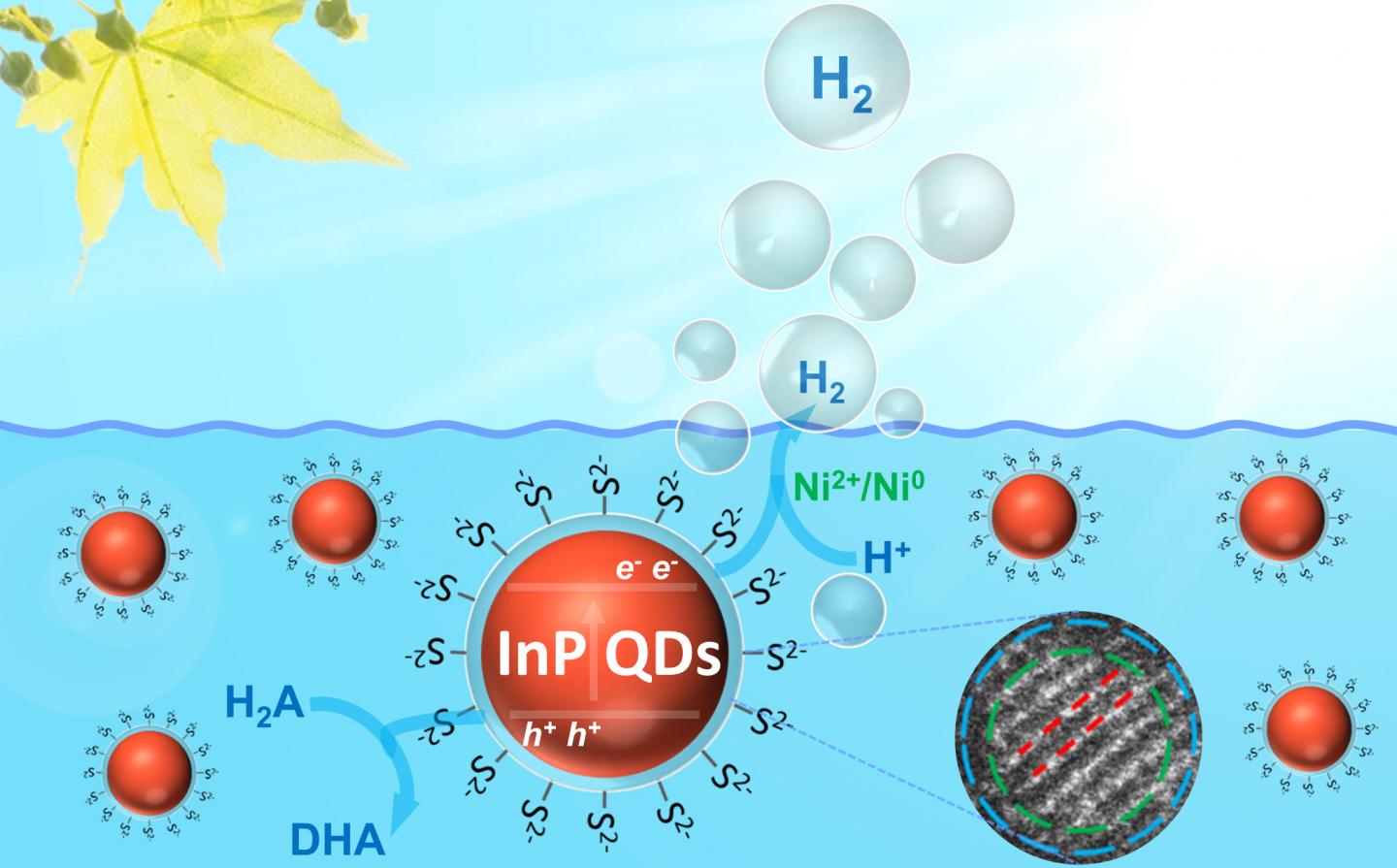
Quantum dots that have been previously used for energy research contain cadmium, which is problematic because the element has been banned from many commodities because of its toxic nature.
A team of consisting of Greta Patzke, Professor at the Department of Chemistry of the University of Zurich, and scientists from the Southwest Petroleum University in Chengdu and the Chinese Academy of Sciences have developed a new type of nanomaterials without any toxic components for photocatalysis. These three-nanometer particles are made of a core of indium phosphide and it is surrounded by a very thin layer of zinc sulfide and sulfide ligands.
“Compared to the quantum dots that contain cadmium, the new composites are not only environmentally friendly, but also highly efficient when it comes to producing hydrogen from light and water,” explained Greta Patzke.
It was found that the sulfide ligands on the quantum dot surface facilitate the crucial steps that are involved in the light-driven chemical reactions, i.e., the efficient separation of charge carriers and their rapid transfer to the nanoparticle surface. These newly developed cadmium-free nanomaterials could be a eco-friendlier alternative in a variety of commercial fields.
“The water-soluble and biocompatible indium-based quantum dots can in the future also be tested in terms of biomass conversion to hydrogen. Or they could be developed into low-toxic biosensors or non-linear optical materials, for example,” added Greta Patzke.
Patzke is going to continue to focus on the development of catalysts for artificial photosynthesis within the University Research Priority Program “LightChEC”.