How did life arise on Earth?- scientists and numerous researches have been and tried to answer this perplexing question. Researches from Rutgers University have now found the evidence that simple protein catalysts, which serve a useful purpose for the functioning of cells- might have existed when life began. This new study of a primordial peptide, also known as a short protein, was published recently in the Journal of the American Chemical Society.
During the late 1980s and early 1990s, chemist Günter Wächtershäuser hypothesized that life began on rocks in the ocean that contained iron and sulfur. Wächtershäuser and others also predicted that short peptides would have bound metals and then played the role of catalysts of life-producing chemistry, according to Vikas Nanda, an associate professor at Rutgers’ Robert Wood Johnson Medical School, who is also the study’s co-author.
Human DNA is made of genes that code for proteins that can be as long as a few hundred to a few thousand amino acids. These proteins are complex, which are essential for all living-things to function properly. They are the result of billions of years of evolution. These proteins were comparatively simpler when life began, maybe only 10 to 20 amino acids long. Nanda says that with the help of computer modeling, scientists at Rutgers University have been trying to figure out how the early peptides might have looked liked what could have been their chemical functions.
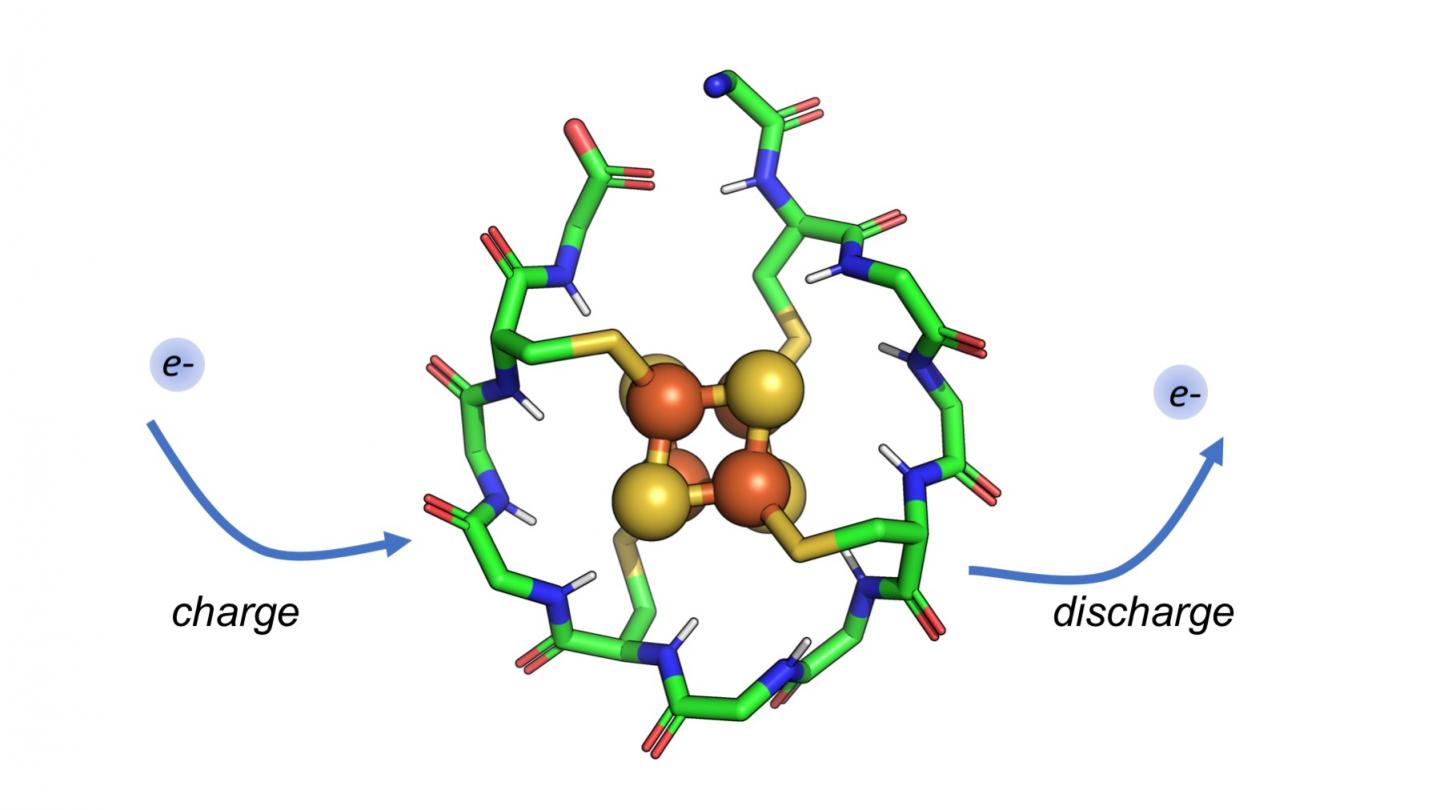
The research team relied on computers to model a short, 12-amino acid protein and tested it in the laboratory. This peptide has various impressive and important feature- it has only two types of amino acids (surprising because there are about 20 amino acids that synthesize millions of different proteins needed for specific body functions).
This peptide is really short and it could have developed spontaneously on early Earth under suitable conditions. There is a metal cluster at the core of this peptide which is close to the structure and chemistry of iron-sulfur minerals that were quite abundant in early Earth oceans. The peptide also has the ability to charge and discharge electrons again and again without falling apart.
“Modern proteins called ferredoxins do this, shuttling electrons around the cell to promote metabolism,” said Professor Paul G. Falkowski, senior authorof the study, who leads Rutgers’ Environmental Biophysics and Molecular Ecology Laboratory. “A primordial peptide like the one we studied may have served a similar function in the origins of life.”